Materials
What you need for THURSDAY:
For each camper:
One medicine cup
One gallon sized baggie
A different brand of diaper (if there is only one camper-there will be two diapers so that a comparison can be made.)
To share:
Bag of Mystery Polymer A
Bag of Mystery Polymer B
Bag of Mystery Polymer C
Measuring cups from your kitchen (optional)
Newspaper, large plastic garbage bag or another material to cover your workspace and make cleaning up easier
Salt
Spoon
Scissors
Introduction
Objectives:
To define what a hydrogel is and how it is used.
To explain how hydrogel monomer units interact to form hydrogel polymers.
To develop and refine observation skills and to generate questions.
To investigate the physical and chemical properties of a hydrogel.
Time Required:
Part 1: 20 minutes
Part 2: 30 minutes
Part 3: 20 minutes
Age Range:
Part 1: Grades 3-8 independently
Part 2: Grades 3-6 with guidance; Grades 7-8 independently
Part 3: Grades 3-8 independently
Background Reading
Background: What is a polymer?
Exerpted from Brittanica Kids https://kids.britannica.com/students/article/polymer/276496
Exerpted from Kids Encyclopedia Facts. https://kids.kiddle.co/Polymer
The term polymer is a composite of the Greek words “poly” and “meros”, meaning “many parts.” Polymers are large molecules made of small, repeating molecular building blocks called monomers. The process by which monomers link together to form a molecule of a relatively high molecular mass is known as polymerization. Many polymer molecules are like chains where the monomer units are the links. Polymer molecules can be straight-chains or they can have cross-linking between chains.
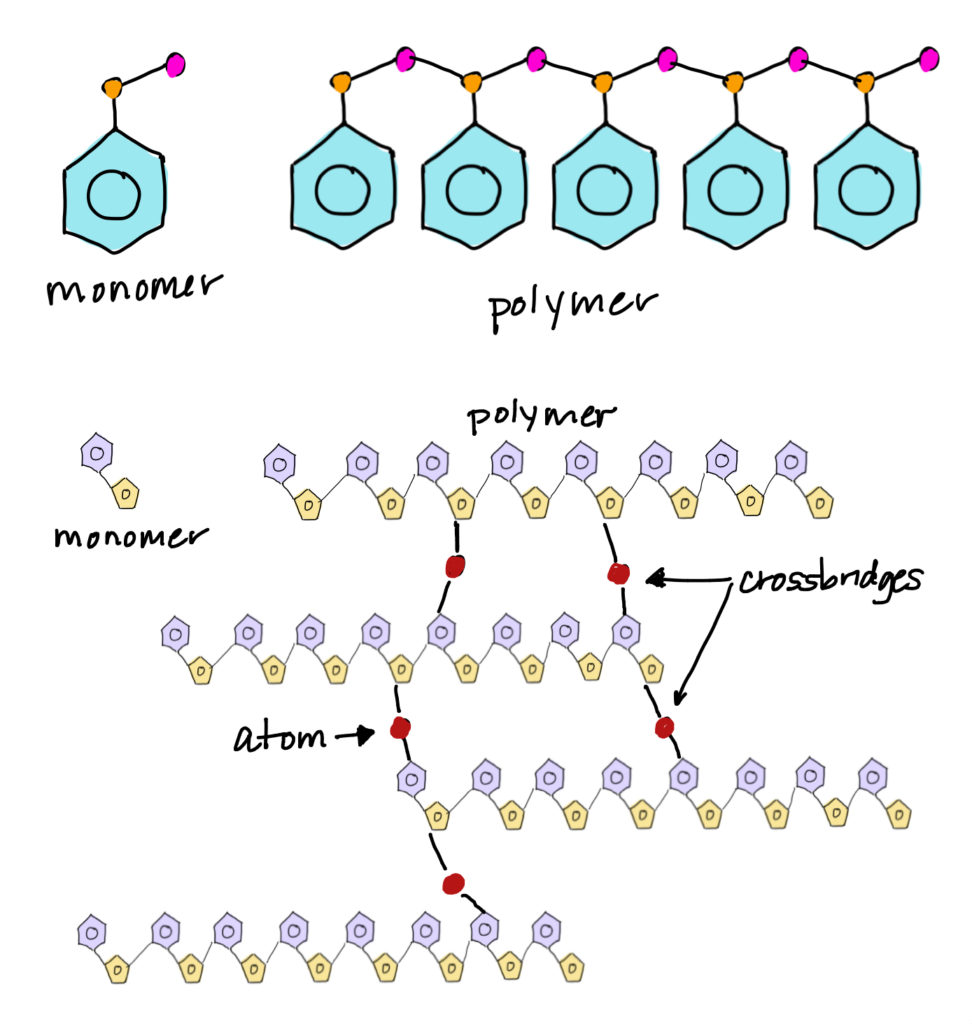
Polymers make up many of the materials in living organisms. Proteins are polymers of amino acids, cellulose is a polymer of sugar molecules, and nucleic acids such as deoxyribonucleic acid (DNA) are polymers of nucleotides. Many synthetic materials, including nylon, paper, plastics, and rubbers, are also polymers.
A variety of simple molecules join together to become useful polymers. The nonstick cookware coating known as Teflon, for example, is made of a monomer composed of two atoms each of fluorine and carbon. Both Plexiglas and Lucite are made of methyl methacrylate, an organic monomer composed of carbon, hydrogen, and oxygen. Silicon polymers used for sealants and other applications are made from inorganic monomers that contain silicon atoms.
Hydrogels are man-made substances that absorb water and hold it in the form of a gel. They’re used in disposable diapers, sanitary pads, breast pads, wound dressings, breast implants, and contact lenses. Hydrogels are also used to thicken products such as bubble bath and lotions and give them a silky, moisturizing feel.
Today, we will be looking at the polymers that make cross-linking chains and are able to hold water. They are called super-absorbant polymers (SAP) and hydrogels.
Do some additional research by exploring “Polymer Science Learning Center” https://www.pslc.ws/macrog/kidsmac/basics.htm
Dissecting a Diaper to Investigate Polymers
Part A: EXPLORE: What is a polymer?
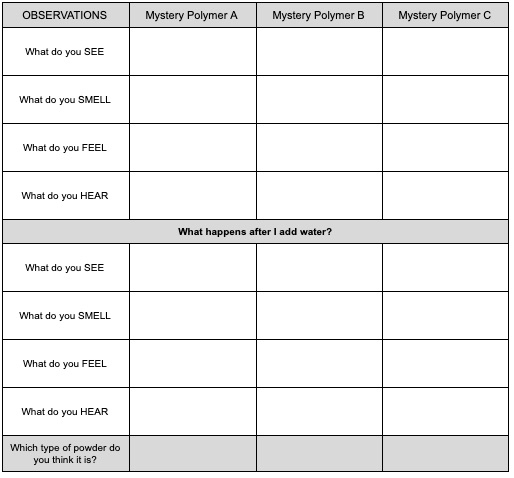
What are hydrogel polymers like? Let’s explore! You have three hydrogel polymers to investigate. Follow the directions to do controlled observations of each of the three polymers.
To share:
Mystery Polymer A
Mystery Polymer B
Mystery Polymer C
Four medicine cups
Water
Procedure:
- Separate three medicine cups. Pour mystery polymer A into the first medicine cup. Pour mystery polymer B into the second medicine cup. Put the mystery polymer C in to the third. Each of these is a type of hydrogel polymer. You should have enough polymer for everyone in the family to do their own exploring.
- Make your observations about each of the powders in your data chart. You should record what you SEE, what you SMELL, what you FEEL, what it SOUNDS like when you touch it and move it around.
- Use the fourth medicine cup to add water to each of the cups. What are your observations as you add the water? Add more water. What happens? Allow the polymer to sit in the water for 5 minutes. Make your observations again. Has anything changed?
- The powders have properties that make them useful for different things. One of the polymers is Instant Snow. One of the polymers is used by florists because it releases water slowly to provide water to flowers. One of the polymers is used in diapers. Which powder is which?
If you are making your own data chart, you can use the suggested design to help you.
If you want to learn more about polymers, try this! https://www.pslc.ws/macrog/kidsmac/basics.htm
Part B: DISSECT: What is the anatomy of a diaper?
To dissect means to separate something into pieces in order to study its internal parts.
A diaper has been designed to keep a baby dry by keeping moisture away from a baby’s skin. The hydrogel most commonly used in diapers is sodium polyacrylate. It’s added to the core of disposable diapers to soak up urine. This helps to prevent diaper rash. The diaper is also designed to prevent urine from leaking out of the diaper to keep the baby’s clothing dry. The anatomy of the diaper is a good example of chemistry and engineering working together to solve a problem. Let’s dissect the diaper and analyze the design!
For each camper:
One gallon sized baggie
One medicine cup (reuse the a medicine cup from Part A)
A brand diaper (if there is only one camper-there will be two brands of diapers to be compared.)
Scissors
To share:
Newpaper, large plastic garbage bag or another material to cover your workspace and make cleaning up easier
Procedure:
- Cover your workspace with newspaper or by a large, plastic garbage bag. This will make cleaning up easier later.
- Place a new diaper on the newly covered surface.
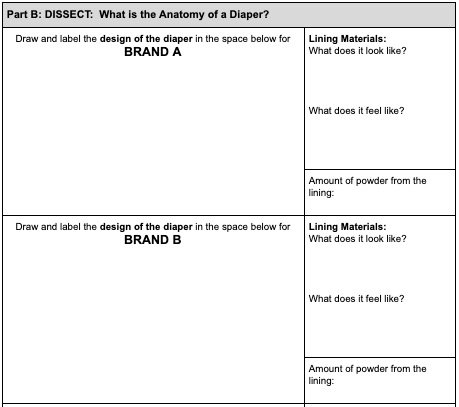
- Make a drawing of the diaper design. Why was it designed this way? Label your observations about the construction.
- Look carefully at the lining. Make some observations about the material used to make the diaper. What does it look like? How does it feel?
- Carefully cut through the diaper lining and remove all of the stuffing material.
- Put all of the stuffing material into your gallon sized plastic bag. Scoop up any material that may have fallen onto your work surface and put it into the plastic bag.
- Seal the bag. Open a small area and blow some air into the bag so that the bag puffs up a little bit.
- Shake the bag for a few minutes. You will begin to see that a powder begins to gather in the bottom of the bag. This is the super absorbent polymer powder.
- Open the bag and while it is still inside the bag, begin to pull apart the cotton. This will help to release any other polymer that is trapped in the cotton.
- Continue to pull at the cotton until you are certain that the majority of the polymer has been released and is now at the bottom of the plastic bag.
- Remove the cotton from the bag. Use the medicine cup to measure the amount of polymer removed from the diaper. Record this amount in your data chart.
- If you have a second diaper, you will need to repeat this procedure again to collect the polymer. Record this amount in your data chart.
INTERESTED IN LEARNING MORE? https://www.youtube.com/watch?v=s6GBfrJd2hs
Part C: INVESTIGATE: How much water can different brands of diapers hold?
There are many brands of diapers. When we dissected your diaper, you removed the sodium polyacrylate from each of the different brand’s diapers. Does one brand work better than another? You can test this out! We want to test our question in a scientific way. This means that we need to do EXACTLY THE SAME THING to the polymer from each diaper.
For each camper:
The polymer that has been removed from the diaper.
Gallon sized plastic bag
To share:
Measuring cups from your kitchen (optional)
Salt
Spoon
Procedure:
We want to know how much water is absorbed by each brand of diaper. You will be deciding on your own method for testing the different diaper brands. How can we test the polymers in a scientific way?
- Put your polymer into its gallon bag. Make sure that you label your bag, so that you remember which diaper you are testing.
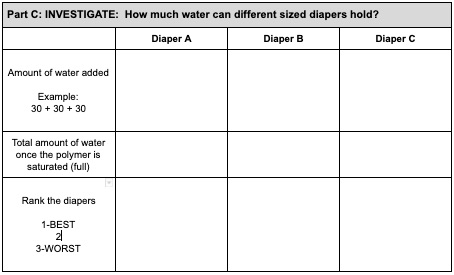
- You will be pouring water into the gallon bag a little bit at a time and mix. Decide on how much water you will add each time. We suggest you add 30ml at a time. You can measure this easily with your medicine cup. If you have measuring cups in your kitchen that you can use, you can measure a different amount each time. But REMEMBER: You will need to add water EXACTLY THE SAME WAY WITH EACH DIAPER. Keep track of how much water you add to the bag by recording it in your data chart.
- Each time you add water, you will need to mix it with the powder. You will need to do this EXACTLY THE SAME WAY WITH EACH DIAPER.
- Continue to add water to the powder in the bag until the powder can no longer hold any more water. If you see liquid water in the bag after mixing it with the powder, that will tell you when to stop. However, you will need to check with one another until you agree that you are done. You will need to see EXACTLY THE SAME WAY WITH EACH DIAPER.
- When you are done, add up the total amount of water added. Which diaper brand absorbed the most? Rank the diapers. (1-BEST, 2, 3-WORST)
To safely clean up the polymer:
Add a few teaspoons of salt, stir it with a spoon, and watch what happens. Salt messes up the gel’s water-holding abilities. When you’re finished, you can pour the saltwater goo safely down the drain.
What Happened?
How do we set up an investigation scientifically?
Whenever we do a science experiment, we follow certain steps to make sure that the experiment will be fair and that it will test what we want it to test. For example: We did an experiment to test whether different brands of diapers hold different amounts of water. We wanted to make sure that our results were just from the brand of diaper and not from some other factor.
When we tested the effect of the diaper brand on the amount of water it can hold, we tested several brands of diaper. The brand of diaper is our manipulated variable. All other variables, (the sodium polyacrylate, the size of the baggie, the way you added the water) would remain the same and we’d call them constants. They have to stay the same for the experiment to be a fair and accurate test. You can imagine what would happen if we just poured some water in the bag for one diaper and didn’t do the same for the second diaper. Or if we mixed the water in the bag for diaper 1 and didn’t mix it in the other bag for diaper 2. We might lose track of the amount of water. The responding variable in this experiment was the amount of water the polymer could absorb. This variable is what we want to observe and record. This makes the comparison more useful to a consumer!
What is happening when we add water to the powder in the diaper?
The sodium polyacrylate is an SAP or superabsorbant polymer. As a polymer, it is a long chain of repeating molecules called monomers. Superabsorbant polymers expand when water is added because the water is pulled into the polymer. The water sticks to the long chains so the sodium polyacrylate acts like a giant sponge. Some SAPs can absorb more than 500 times their weight in water!
The polymer is spread out in the center of the diaper using the cotton fibers that you dissected out of the diaper. This will make sure that the water is absorbed evenly across the diaper. Otherwise, as the baby filled the diaper, the diaper would get lumpy and uncomfortable for the baby to wear. Even the smallest amount of powder will hold a lot of water so the diaper design will help to keep the baby dry.
The polymers in diapers can be a problem if we don’t remember that the powders are so absorbant! A diaper that is worn in a swimming pool will quickly absorb lot of water. If they fill too much, the diaper will break open and the polymer will leak into the pool water. This is not good for the filtering system of the pool. Some people will flush diapers down the toilet. What do you think will happen as diaper moves with the water through the pipes?

