CORONAVIRUS
The Life Sciences Community is Leading the Charge in the Fight Against CoronavirusThe Coronavirus is a serious global public health threat with information rapidly evolving. Innovative Life Sciences Companies are ramping up efforts to study the virus and develop solutions to prevent, diagnose and treat this deadly infectious disease.
Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). Coronavirus disease (COVID-19) is a new strain that was discovered in 2019 and has not been previously identified in humans.
Life Sciences Companies are responding faster than ever to emerging health threats. We are proud of our Members who are working hard to address the threat of COVID-19 on behalf of patients around he world.
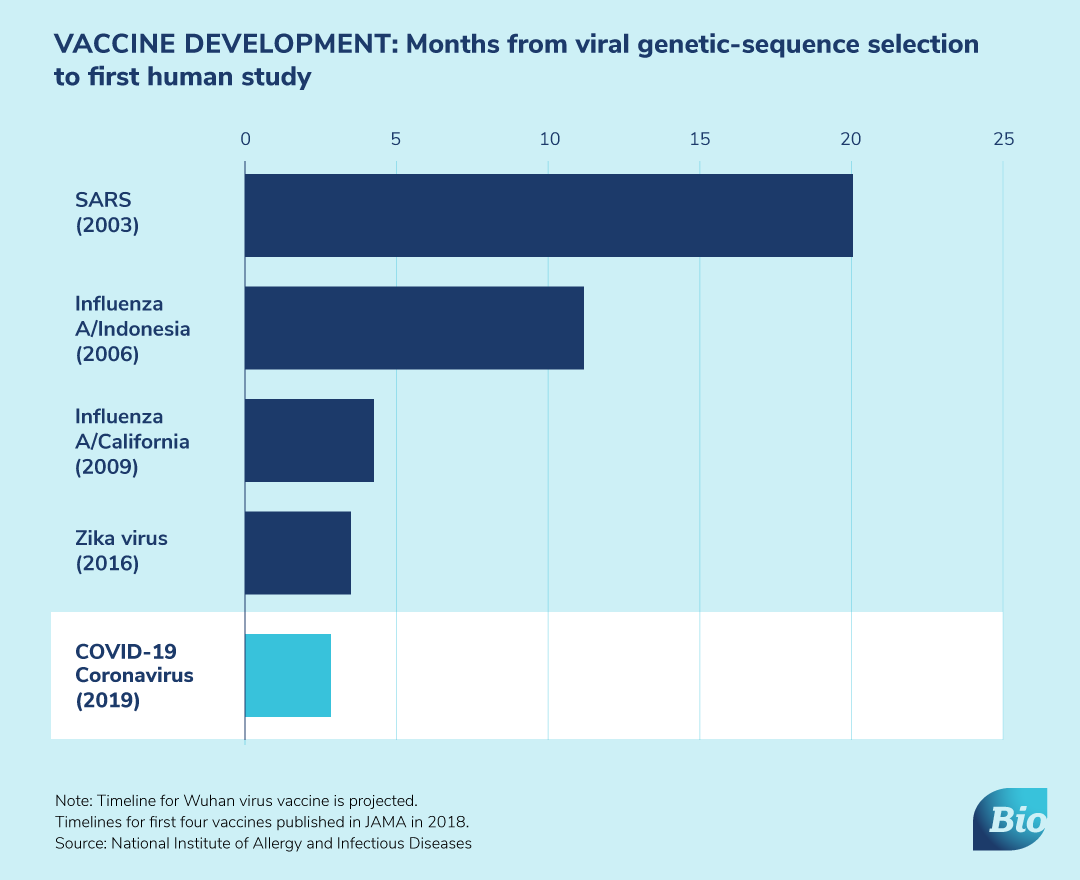
Here is how our members are responding:
What you need to know: Illinois Expanded COVID-19 Vaccine Distribution Plan
Building on guidance by the Centers for Disease Control and Prevention's (CDC) Advisory Committee on Immunization Practices (ACIP) and the Illinois Department of Public Health (IDPH), Governor JB Pritzker announced guidelines for the next stage of COVID-19 vaccine...
iBIO Statement on the FDA’s Emergency Use Authorization for Pfizer’s COVID-19 Vaccine
“Today marks a turning point in our fight against the COVID-19 pandemic. The U.S. Food and Drug Administration has issued an emergency use authorization, EAU, for the Pfizer-BioNTech COVID-19 vaccine. iBIO commends the researchers, doctors, manufacturing employees,...
LIVE STREAM: FDA VACCINES AND RELATED BIOLOGICAL PRODUCTS ADVISORY COMMITTEE MEETING
The U.S. Food and Drug Administration has scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Dec. 10 to discuss the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, Inc. in partnership...
FDA Guidance:
FDA Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency
The Food and Drug Administration (FDA or Agency) plays a critical role in protecting the United States from threats including emerging infectious diseases, including the Coronavirus Disease 2019 (COVID-19) pandemic. FDA is committed to providing timely guidance to...
FDA Temporary Policy for Manufacture of Alcohol for Incorporation Into Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry
This guidance is being issued to address the Coronavirus Disease 2019 (COVID-19) public health emergency. This is being implemented without prior public comment because FDA has determined that prior public participation for this guidance is not feasible or appropriate...
BIO COVID-19 Therapeutic Development Tracker
iBIO Resource Center:
iBIO Community Coronavirus Survey Results:
As concerns about the Coronavirus were beginning to escalate, iBIO conducted a survey of our member companies to catalog policies and practices to protect employee safety and safeguard operations.
The survey shows that companies immediate focus is on ensuring the health and safety of their employees, and reducing the spread of the coronavirus.
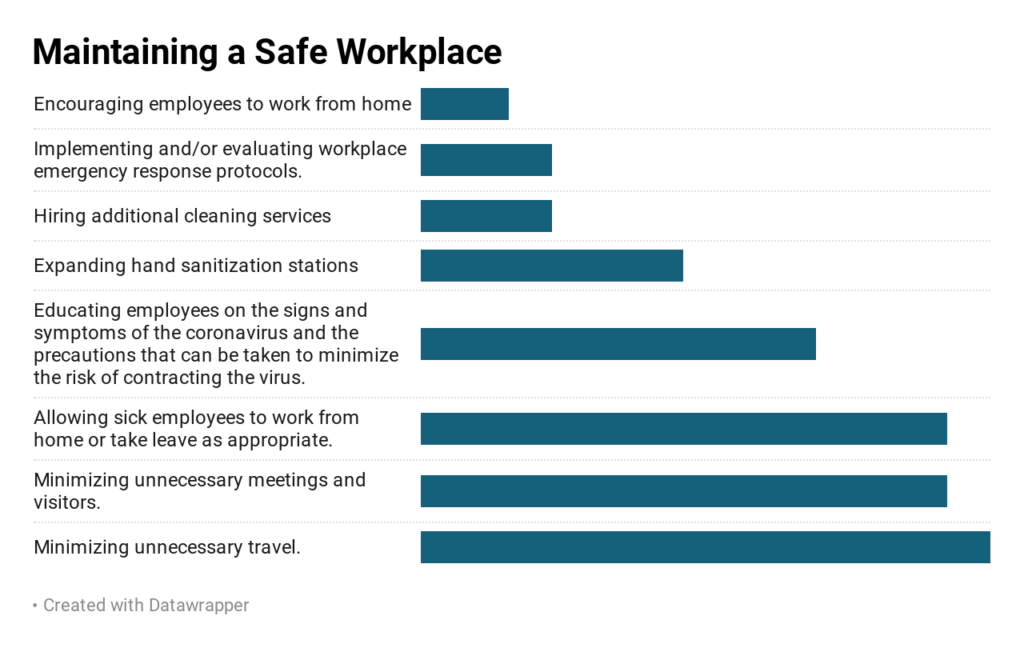
All of the companies responding to iBIO’s survey have instituted restricted travel policies and over 90% of the companies have reduced unnecessary external meetings and allow sick employees to work from home or take leave as appropriate. Since Monday, 15% of the responding companies updated their responses to include encouraging employees to work from home.
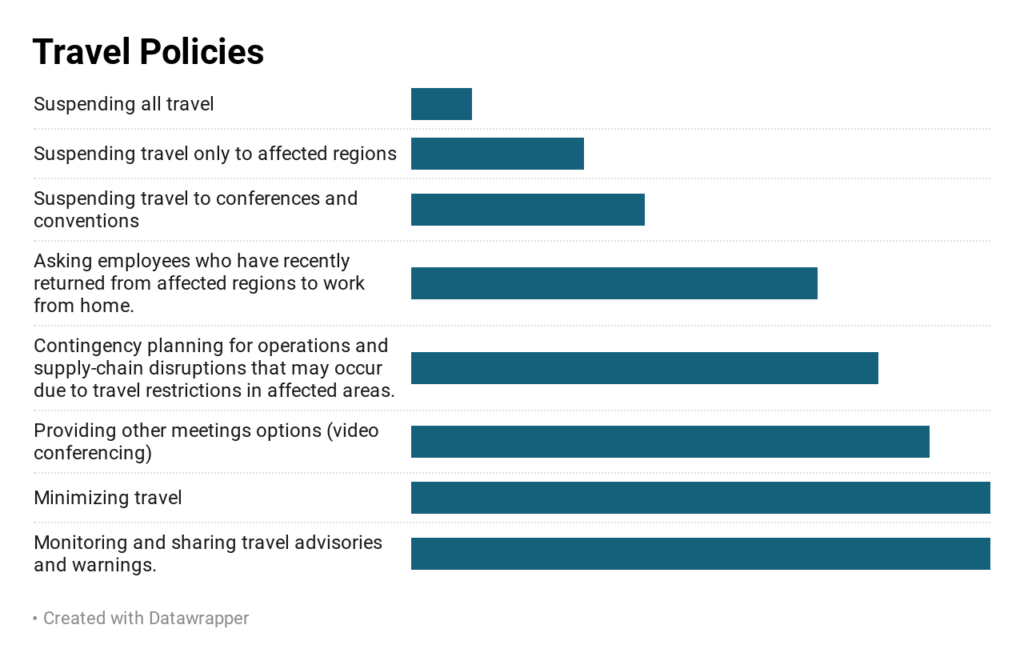
All of the responding companies have suspended or restricted travel to affected areas. Most of the companies have also restricted travel to conferences and over half of the companies are requiring employees returning from affected areas to work from home. Over 60% of member companies are also providing alternative meeting options like video conferencing for their employees.
Currently none of the companies have reported any disruptions to their supply chain but 60% of the companies responded that they have concerns about the impact on their operations and are developing contingency plans for operational and supply-chain disruptions that may occur due to their restricted travel policies. 10% of the responding companies are anticipating clinical trial disruptions.
Best practices in developing a corporate strategy and plan.
Provided the importance of people to every organization, Deloitte has developed a framework for companies to plan for employee and operational needs during this unfolding challenge.
Deloitte’s framework focuses on three connected dimensions of a company: work (operations), workforce (employees) and workplace (offices and meetings). Here are some of the practices and strategies for consideration.
WORK
- Establish a business response and continuity office made up of a cross-functional team to develop coordinated response effort for the organization. This team should determine meaningful organizational activation and deactivation triggers, and review continuity procedures to better understand critical staff, functions, and operational hubs.
- Confirm critical roles and backup plans. Prepare temporary succession plans for key executive positions and critical roles in the organization. In the event of illness, your board and management team need to have clear leadership alternatives.
- Evaluate the actual work conducted by your organization and how it might be changed. Identify what work requires on-site attendance. Many companies are shifting face-to-face meetings to teleconferences to reduce the risk of virus transmission. A combination of technologies (e.g., remote access, cyber), practices and policies, safeguards, and training all need to be in place to support a wide remote-work deployment. For work that cannot be made remote, evaluate what safeguards can be put in place, such as revised cleaning protocols or personal protective equipment.
- Prioritize mission critical operations and identify projects and operations that can be deferred or deprioritiezed.
- Supply Chain Shortages. The FDA has been monitoring the drug supply chain and communicating with manufacturers related to the possibility of shortages of drugs, API, and related supplies. On February 25th, the FDA announced it had identified 20 drugs at risk of shortage. As of February 28th, FDA has reported a shortage of one drug as a result of the ongoing COVID-19 outbreak. Manufacturers of all prescription drugs are required to notify the FDA of a permanent discontinuance or temporary interruption in manufacturing that is likely to lead to a meaningful disruption in the supply of a covered drug in the United States. The notification is required six months in advance, or if that is not possible, as soon as practicable, but no later than 5 business days after the discontinuance or interruption in manufacturing. Shortages or supply issues should be reported to the FDA through the CDER Direct NextGen Portal. Additional information on reporting shortages or supply issues is available from the FDA.
WORKFORCE
- Set the tone from the top. Proactive and consistent messaging and modeling behavior from executives is critically important to employee moral. This situation is not permanent and while there may be disruption, there will also be recovery.
- Develop a plan for the whole workforce. Employers should not forget about their contract and vendor workforce. Identify crucial contributors to the company’s operations and ensure they are included in your company’s plans to keep your workforce safe.
- Make your communications strategy visible. Outline communication plans with your leaders so they know what to expect and what their role is.
- Educate employees about COVID-19 symptoms, prevention measures and establish employee support procedures. Companies should develop dedicated communication systems to handle inquiries from employees.
- Develop workforce plans and scenario analysis. Think ahead to how this situation could play out, including the recovery period. Companies should consider in advance how to restart disrupted business operations amid ongoing epidemic prevention and control measures. Keep in mind that quarantines and travel restrictions could mean the time to ramp back up to full capacity may be longer–and more complicated–than after a planned shutdown.
- Review leave policies and consider whether eligible criteria should be temporarily changed during this period.
WORKPLACE
- Prepare the workplace. Companies should ensure the safety of working environments by thoroughly cleaning and disinfecting workplaces. In the event that an employee is suspected of being infected with COVID-19, a clear process must be in place for removing that employee from the facility, and for proper treatment of the facility.
- Update travel and meeting protocols. For organizations with high travel needs, especially to international destinations, assessing the impact of the epidemic on travel is necessary as travel has been linked to the transmission of COVID-19.
- Review your social media policy and guidelines. Make sure your social media policy is properly defined for this crisis. It should provide clear guidelines with regard to how employees can talk about your business and the impacts of COVID-19 on operations and employee health and safety. Provide employees with an internal channel to report what they are seeing and feeling within the organization to ensure direct communication as much as possible as an alternative to social media.
- Consider the sources of ‘news’ and information in the workplace. Misinformation in the media has created particular challenges for organizations responding to this outbreak. Employers must take it upon themselves to be the source of accurate, timely, and appropriate information for their workforce. Consider creating your own news channel in the workplace, appropriately filtered and based on credible sources.




